Understand the basics of charges, how ions are formed, their types, key differences, and a comprehensive reference table of cations and anions.”
We Will Learn :
- What is a Charge?
- How Charging Happens?
- What is an Ion?
- Difference Between Charges and Ions
- Types of Ions (based on charge & structure)
- Key Points to Remember
- Table of ions
- List of Elements with Their Ion Charges
- Common Molecular (Polyatomic) Ions
What is a Charge?
- A charge is a property of particles (like protons, electrons, and ions) that causes them to attract or repel each other.
- There are two types of charges:
- Positive charge (+): Due to loss of electrons.
- Negative charge (−): Due to gain of electrons.
- Neutral atoms have no charge (equal protons & electrons).
How Charging Happens?
Atoms gain or lose electrons to achieve a stable electronic configuration (usually like noble gases).
- Loss of electrons → Positive ion (cation)
- Gain of electrons → Negative ion (anion)
Example:
- Sodium (Na) → Na⁺ (by losing 1 electron)
- Chlorine (Cl) → Cl⁻ (by gaining 1 electron)
Remember :
- Electron donating atom is called “Donor” and
- Electron accepting atom is called “Acceptor”.
What is an Ion?
- An ion is a charged particle formed when an atom or group of atoms gains or loses electrons.
- Types of ions:
- Cations (positive ions): Metals usually form cations.
- Anions (negative ions): Non-metals usually form anions.
- Monoatomic ions: Single atom ions (Na⁺, Cl⁻, O²⁻).
- Polyatomic ions: Group of atoms with a charge (SO₄²⁻, NH₄⁺, CO₃²⁻).
Difference Between Charges and Ions
| Aspect | Charge | Ion |
|---|---|---|
| Definition | Property of matter (positive/negative/zero) | Atom or group of atoms carrying a charge |
| Types | + (positive), – (negative), 0 (neutral) | Cations (+), Anions (–), Monoatomic, Polyatomic |
| Existence | Abstract property (no particle itself) | Real particle (Na⁺, Cl⁻, SO₄²⁻, etc.) |
| Example | +1, –2 | Na⁺, Cl⁻, O²⁻, NH₄⁺, SO₄²⁻ |
Types of Ions (based on charge & structure)
- Cations: Na⁺, Ca²⁺, Al³⁺
- Anions: Cl⁻, O²⁻, SO₄²⁻
- Monoatomic ions: K⁺, Fe²⁺, Br⁻
- Polyatomic ions: NO₃⁻, CO₃²⁻, NH₄⁺
Key Points to Remember
- Metals → form cations (+).
- Non-metals → form anions (–).
- Noble gases → mostly do not form ions (stable).
- Transition elements → can have multiple oxidation states (like Fe²⁺, Fe³⁺).
- Polyatomic ions → act as a single charged unit in compounds.
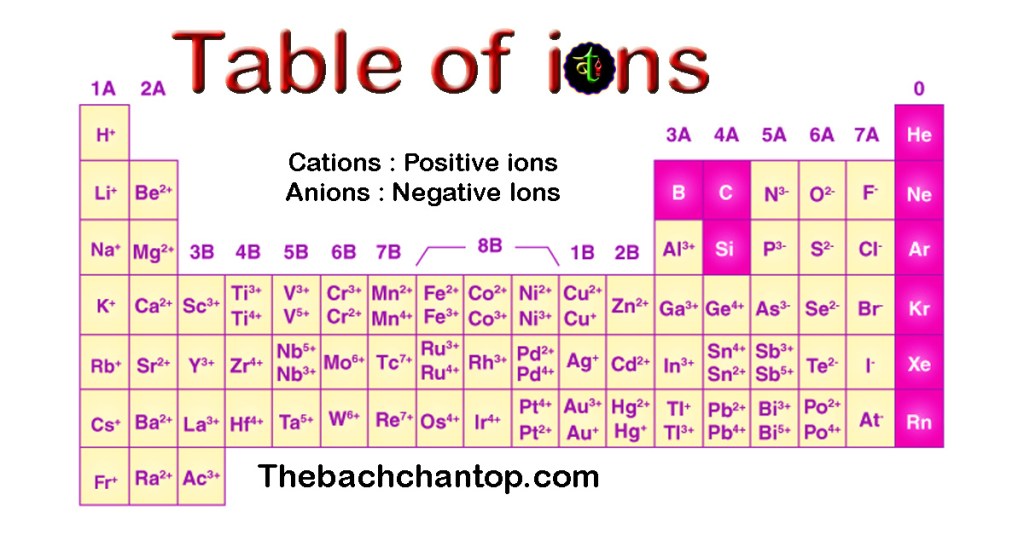
Table of ions

List of Elements with Their Ion Charges
| S.No | Ion Name | Symbol / Formula |
|---|---|---|
| 1 | Hydrogen | H⁺ |
| 2 | Helium | He (no ion) |
| 3 | Lithium | Li⁺ |
| 4 | Beryllium | Be²⁺ |
| 5 | Boron | B³⁻, B³⁺ |
| 6 | Carbon | C⁴⁺ |
| 7 | Nitrogen | N³⁻ |
| 8 | Oxygen | O²⁻ |
| 9 | Fluorine | F⁻ |
| 10 | Neon | Ne (no ion) |
| 11 | Sodium | Na⁺ |
| 12 | Magnesium | Mg²⁺ |
| 13 | Aluminium | Al³⁺ |
| 14 | Silicon | Si⁴⁺, Si⁴⁻ |
| 15 | Phosphorus | P⁵⁺, P³⁺, P³⁻ |
| 16 | Sulphur | S²⁻, S²⁺, S⁴⁺, S⁶⁺ |
| 17 | Chlorine | Cl⁻ |
| 18 | Argon | Ar (no ion) |
| 19 | Potassium | K⁺ |
| 20 | Calcium | Ca²⁺ |
| 21 | Scandium | Sc³⁺ |
| 22 | Titanium | Ti⁴⁺, Ti³⁺ |
| 23 | Vanadium | V²⁺, V³⁺, V⁴⁺, V⁵⁺ |
| 24 | Chromium | Cr²⁺, Cr³⁺, Cr⁶⁺ |
| 25 | Manganese | Mn²⁺, Mn⁴⁺, Mn⁷⁺ |
| 26 | Iron | Fe²⁺, Fe³⁺ |
| 27 | Cobalt | Co²⁺, Co³⁺ |
| 28 | Nickel | Ni²⁺ |
| 29 | Copper | Cu⁺, Cu²⁺ |
| 30 | Zinc | Zn²⁺ |
| 31 | Gallium | Ga³⁺ |
| 32 | Germanium | Ge⁴⁻, Ge²⁺, Ge⁴⁺ |
| 33 | Arsenic | As³⁻, As³⁺, As⁵⁺ |
| 34 | Selenium | Se²⁻, Se⁴⁺, Se⁶⁺ |
| 35 | Bromine | Br⁻, Br⁺, Br⁵⁺ |
| 36 | Krypton | Kr (no ion) |
| 37 | Rubidium | Rb⁺ |
| 38 | Strontium | Sr²⁺ |
| 39 | Yttrium | Y³⁺ |
| 40 | Zirconium | Zr⁴⁺ |
| 41 | Niobium | Nb³⁺, Nb⁵⁺ |
| 42 | Molybdenum | Mo³⁺, Mo⁶⁺ |
| 43 | Technetium | Tc⁶⁺ |
| 44 | Ruthenium | Ru³⁺, Ru⁴⁺, Ru⁸⁺ |
| 45 | Rhodium | Rh⁴⁺ |
| 46 | Palladium | Pd²⁺, Pd⁴⁺ |
| 47 | Silver | Ag⁺ |
| 48 | Cadmium | Cd²⁺ |
| 49 | Indium | In³⁺ |
| 50 | Tin | Sn²⁺, Sn⁴⁺ |
| 51 | Antimony | Sb³⁻, Sb³⁺, Sb⁵⁺ |
| 52 | Tellurium | Te²⁻, Te⁴⁺, Te⁶⁺ |
| 53 | Iodine | I⁻ |
| 54 | Xenon | Xe (no ion) |
| 55 | Cesium | Cs⁺ |
| 56 | Barium | Ba²⁺ |
| 57 | Lanthanum | La³⁺ |
| 58 | Cerium | Ce³⁺, Ce⁴⁺ |
| 59 | Praseodymium | Pr³⁺ |
| 60 | Neodymium | Nd³⁺, Nd⁴⁺ |
| 61 | Promethium | Pm³⁺ |
| 62 | Samarium | Sm³⁺ |
| 63 | Europium | Eu³⁺ |
| 64 | Gadolinium | Gd³⁺ |
| 65 | Terbium | Tb³⁺, Tb⁴⁺ |
| 66 | Dysprosium | Dy³⁺ |
| 67 | Holmium | Ho³⁺ |
| 68 | Erbium | Er³⁺ |
| 69 | Thulium | Tm³⁺ |
| 70 | Ytterbium | Yb³⁺ |
| 71 | Lutetium | Lu³⁺ |
| 72 | Hafnium | Hf⁴⁺ |
| 73 | Tantalum | Ta⁵⁺ |
| 74 | Tungsten | W⁶⁺ |
| 75 | Rhenium | Re²⁺, Re⁴⁺, Re⁶⁺, Re⁷⁺ |
| 76 | Osmium | Os³⁺, Os⁴⁺, Os⁶⁺, Os⁸⁺ |
| 77 | Iridium | Ir³⁺, Ir⁴⁺, Ir⁶⁺ |
| 78 | Platinum | Pt²⁺, Pt⁴⁺, Pt⁶⁺ |
| 79 | Gold | Au⁺, Au²⁺, Au³⁺ |
| 80 | Mercury | Hg⁺, Hg²⁺ |
| 81 | Thallium | Tl⁺, Tl³⁺ |
| 82 | Lead | Pb²⁺, Pb⁴⁺ |
| 83 | Bismuth | Bi³⁺ |
| 84 | Polonium | Po²⁺, Po⁴⁺ |
| 85 | Astatine | At⁻ |
| 86 | Radon | Rn (no ion) |
| 87 | Francium | Fr⁺ |
| 88 | Radium | Ra²⁺ |
| 89 | Actinium | Ac³⁺ |
| 90 | Thorium | Th⁴⁺ |
| 91 | Protactinium | Pa⁵⁺ |
| 92 | Uranium | U³⁺, U⁴⁺, U⁶⁺ |
Common Molecular (Polyatomic) Ions
| S.No | Ion Name | Symbol / Formula |
|---|---|---|
| 1 | Ammonium | NH₄⁺ |
| 2 | Hydroxide | OH⁻ |
| 3 | Nitrate | NO₃⁻ |
| 4 | Nitrite | NO₂⁻ |
| 5 | Sulfate | SO₄²⁻ |
| 6 | Sulfite | SO₃²⁻ |
| 7 | Thiosulfate | S₂O₃²⁻ |
| 8 | Carbonate | CO₃²⁻ |
| 9 | Bicarbonate (Hydrogen carbonate) | HCO₃⁻ |
| 10 | Cyanide | CN⁻ |
| 11 | Acetate (Ethanoate) | CH₃COO⁻ |
| 12 | Permanganate | MnO₄⁻ |
| 13 | Dichromate | Cr₂O₇²⁻ |
| 14 | Chromate | CrO₄²⁻ |
| 15 | Phosphate | PO₄³⁻ |
| 16 | Hydrogen phosphate | HPO₄²⁻ |
| 17 | Dihydrogen phosphate | H₂PO₄⁻ |
| 18 | Perchlorate | ClO₄⁻ |
| 19 | Chlorate | ClO₃⁻ |
| 20 | Chlorite | ClO₂⁻ |
| 21 | Hypochlorite | ClO⁻ |
| 22 | Perbromate | BrO₄⁻ |
| 23 | Bromate | BrO₃⁻ |
| 24 | Iodate | IO₃⁻ |
| 25 | Periodate | IO₄⁻ |
| 26 | Oxalate | C₂O₄²⁻ |
| 27 | Peroxide | O₂²⁻ |
| 28 | Silicate | SiO₃²⁻ |
| 29 | Arsenate | AsO₄³⁻ |
| 30 | Borate | BO₃³⁻ |
Must Read :
- How Is Science Different from Technology? | विज्ञान और तकनीक में क्या अंतर है?
- Why Is Observation Important in Science? | विज्ञान में अवलोकन क्यों आवश्यक है?
- What Is Applied Science? | प्रायोगिक विज्ञान क्या है?
- What Is Pure Science? | शुद्ध विज्ञान क्या है?
- What Is Physical Science? | भौतिक विज्ञान क्या है?
- What Is Biological Science? | जैविक विज्ञान क्या है?
- What Is Earth Science? | पृथ्वी विज्ञान क्या है?

Leave a comment